

Here you get preparation assistance for various competitive examinations in the form of Test Series, Online Classes, Quizzes, and many more.The bleach dilution you need depends mostly on its application - what do you want to use it for?
#GRAMS TO LITERS CHEMISTRY CALCULATOR DOWNLOAD#
For more informative and educational content like this, you can download the free Testbook App. We hope the free online tool in the Grams to Moles Calculator has been useful to you. In one mole of glucose, we have six carbon atoms, 12 hydrogen atoms and six oxygen atoms. The molecular formula of glucose is C6H12O6 The Atomic weight of hydrogen is 1 g/mol and In one mole of water, we have two Hydrogen and one Oxygen atom. Solved Examples on Grams to Moles Calculation The units of measurement in the CGS system are. It was introduced because the units were easy to use for electrical, magnetic, and thermal energy calculations.įor example, the heat capacity of water is 1 calory per degree celsius. The Centimeter-Gram-Second system or simply the CGS system of measurement was proposed by German mathematician Carl F. The unit Gram is from the CGS system of units. In chemistry, the substances are measured in grams and milligrams for our convention purpose in treating the materials. Gram is a metric unit of mass which is defined as one-thousandth of a kilogram. ‘mol’ is used to denote mole in practical problems. In essence, that is the reason this specific unit was created. Anytime you want to to make a reference to a lot of different things, it is much simpler to type the word "mole" rather than the number 6.023*1023. 6.023*1023 carbon atoms make up a mole of carbon. For your reference 6.023*1023 is Avogadro's Number. It is the SI unit to measure the amount of any substance.įor all intents and purposes, the mass of one mole of a substance in grammes and the mass of one molecule in daltons are essentially equivalent.Ī mole was once defined as the volume of something that has the same number of particles as 12.000 grams of Carbon-12. Any substance is made up of a large number of atoms, molecules, or other components, the mole is a useful measure to utilise in this case. This is the reason why we convert given grams of substance into moles and this gram to moles calculator tool by Testbook aids that purpose.Ī mole is 6.023*1023 of any chemical unit, including atoms, molecules, ions, and others. The earlier approach (mole approach) helps us with the same. The latter approach (using mass) provides no information regarding the proportion of each species' quantities that react to produce the given amount of liquid water. With the 1st approach, here we have 2 moles of hydrogen reacting with 1 mole of oxygen to produce 2 moles of water.Īs per the 2nd approach, if we consider mass, we have 4g of Hydrogen gas in reaction with 32g of Oxygen gas to result in 36g of liquid water. To analyze the above equation, we have two approaches - using mole and unison mass. Let’s go through the example given below for a better understanding of mass and moles in a chemical reaction.

The number of atoms, molecules, or ions that are actually reacting to produce a specific number of atoms, molecules, or ions is not indicated by the use of mass alone. It would be convenient to know the precise ratio of the amounts of each species when examining reactants and products in a chemical process. Why do we use the Grams to Moles Calculator? Step 3: In the output field, the grams to moles conversion will be shown.
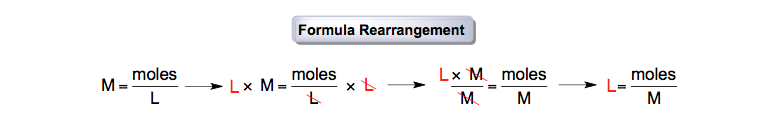
Step 2: To obtain the conversion value, click the "Solve" button now. Step 1: Fill in the input field with the grams and formula weight. Testbook’s grams to moles converter should be used as follows: You can convert grams to moles calculator with the steps mentioned below.
#GRAMS TO LITERS CHEMISTRY CALCULATOR MANUAL#
Along with the free online calculator tool, the article also focuses on manual methods to convert grams into moles, solved examples and some brainstorming FAQs. We got you! Testbook provides you with this facility with the help of grams to moles calculator which is super quick and easy to use. Converting grams into moles can be tricky.


 0 kommentar(er)
0 kommentar(er)
